Welcome to lies by omission, brought to you by the little faith in our educational system. You know which kind of lies I’m referring to, those similar to the ones your partner doesn’t tell you, so technically, they aren’t lying? Yep, that’s it. How can we truly be excited when we hear scientific breakthroughs, such as the discovery of the Higgs Boson, when most people have no idea that there is so much more in the world of subatomic particles that play important roles in our everyday life? This will be just the tip, an overview of all the parts that matter in an atom. More specifically, the elementary particles of an atom.
All of matter is made up of atoms. Energy, photons, that’s something else. But matter: solid, liquid, gas, and plasma all interact with electromagnetic forces and follow the Pauli Exclusion Principle. This principle essentially allows all energy shells to be occupied and allows electrons to create bonds with other atoms, creating new molecules, and essentially makes us all, not Helium. Or, more scientifically, it states that no two identical half integers spin particles can occupy the same quantum state.
As we knew it, or the majority know we have protons, neutrons, and electrons, the OG’s (original gangsters), if you will. Well, since 1932, at least, when James Chadwick discovered the last subatomic particle, the neutron. Their positioning and image resembled that of the solar system, remember? Protons and neutrons are in the nucleus (center), while the electron orbits around it—the good old days. Our journey from Aristotle to the present day was complete; this is as far as we can go. NO FURTHER. Well, turns out the left out a quiet a bit.
Below is a little image to jog your memory of the aforementioned subatomic particles and, hopefully, your love of chemistry. I kid, I know physics isn’t the global favorite that biology is.
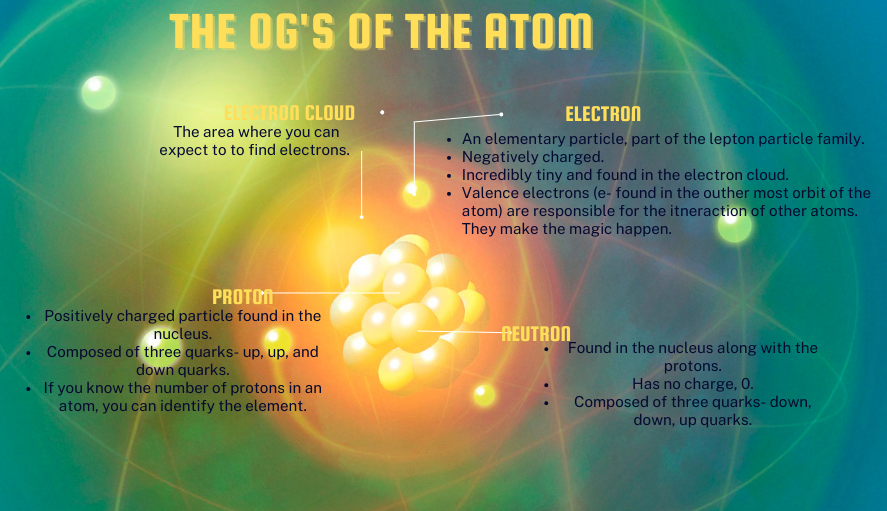
Not till 1932 did we have all three of those particles in our grasp and understanding of their charge. Then, in 1964, almost 30 years later, Murray Gell-Man experimented with colliding protons with other protons and electrons at high speed, which led to the discovery of even smaller particles! Quarks. Tip on pronunciation, it’s read kwork.
All this time, protons and neutrons were holding inside of them even smaller bundles of joy-quarks! Lo and behold, there are six different kinds of quarks, known as flavors. Now, protons and neutrons have three quarks each, which is necessary to be stable. These six flavors have incredibly adorable names: up, down, charm, strange, top, and bottom. Yep, those are their names.
So now our atom party has progressed- the new elementary particles in an atom are the electrons and the quarks. Now here comes the absolutely fun part… math! Unfortunately, math is everywhere. Anyone who says otherwise is living in denial, and congratulations.
As you know from the image of the atom, each particle has a charge. Protons have a positive charge, electrons have a negative charge, and neutrons, well, they have no charge (a strong reason why they were the last to be discovered, they were incognito!). So how can we calculate their charge? Well, these adorable quarks have charges!
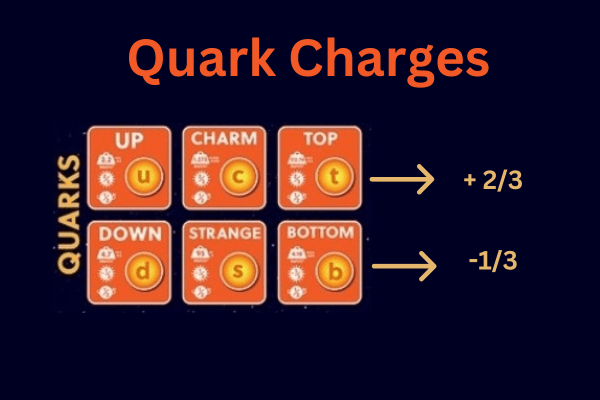
Protons consist of an up, up, and down quark.
Neutrons consist of an up, down, and down quark.
How’s the math looking? Perfect! The charges respectively amount to 1 and 0.
One last thing before we digest the beauty of quarks in atom-both electrons and quarks belong to a larger group of particles known as fermions. Fermions consist of two groups: quarks and leptons. Fermions are what make up the matter in the universe. So when we talk about matter, I’m referring to fermions. We just superficially covered the quarks that play a role in the atom. Next, I’ll speak of the leptons. But don’t worry; I’ll keep it to the electron…mostly.
Source
Hawking, Stephen, 1942-2018. A Brief History of Time. New York. Bantam Books, 1998.
